How do we make cattle cloning more efficient?

Case study: Improving cattle cloning efficiencies.
Cloning has the potential to improve cattle breeding. However only a few per cent of cloned embryos produced using skin cells from live animals produce calves, which limits its use commercially. Instead of using skin cells we can make stem cells from these which we are using to increase cloning efficiencies.
The aim of this project was to demonstrate that a new stem cell type isolated by University of Adelaide scientists can be used to improve cloning efficiencies in cattle.
Current cattle cloning efficiencies are relatively low compared with other breeding technologies such as embryo transfer, which limits its use commercially. Cloning involves the insertion of a skin cell (fibroblast) into an unfertilised egg or oocyte, which has had its genetic material removed.
However only around 2% of embryos are sufficiently reprogrammed to allow them to develop to term. Previous work in mice has suggested that the use of embryonic stem cells can increase cloning efficiencies by up to 10 fold1. Embryonic stem cells are isolated from embryos and require less reprogramming than fibroblasts.
However ESCs are yet to be isolated for any of the livestock species preventing the use of this approach for improving cloning. In a breakthrough we have isolated a new type of embryonic stem cell in pigs, cattle and mice2 (Inner cell mass stem cells (ICMSCs)).
In other work we have shown that these cells can also be isolated from cloned embryos produced using skin cells taken from live males or females3.
The aim of the present study is to determine whether these cells can be used to improve cloning efficiencies in cattle as shown in Figure 2. These cells will also be useful for other applications including gene discovery using gene editing.
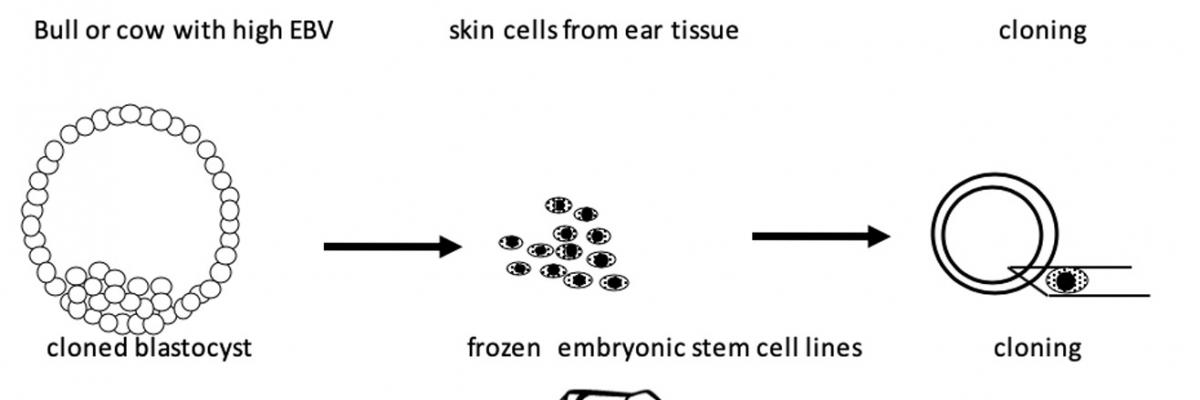
Fig 2. Improving current cattle cloning efficiencies using embryonic stem cells. Skin cells isolated from the ear tissue of live animals are used to produce cell lines which are then frozen. Fibroblasts are then used for cloning to produce embryos from which embryonic stem cells are then isolated and frozen. These are then used for cloning to increase efficiencies.
Progress
- Isolate new embryonic stem cell lines for this work - Three porcine lines were isolated and characterised (we are using pigs as a model initially because of a temporary lack of cattle oocytes).
- Determine fusion and activation parameters for these cells for cloning - Currently we are determining the best way to fuse these cells to oocytes as well as activate these embryos. Several approaches have are being examined including injecting cells into oocytes.
- As part of work we have also demonstrated that addition of Granulocyte Macrophage Colony Stimulating Factor (GM-CSF) a cytokine found in the ovary, to our embryo culture media increases the proportion of embryos that produce stem cell lines. Possibly due to its effect in increasing the number of cells in the embryo that give rise to the fetus. As such we are also examining whether GM-CSF can be used to in vitro embryo production (IVP) success rates in a separate project aimed at raising awareness and adoption of this technology by breeders.
Future work
Having optimised methods for fusing and activating these cells, the next step is to demonstrate that ICMSCs increase cloning efficiencies by examining embryo development and then birth rates.
The study will also examine differences in gene expression between cloned embryos produced using or stem cell type and fibroblasts as a measure of reprogramming success.
Ultimately we aim to determine what are the factors in the oocyte that reprogram the donor nucleus so that these can be used to reprogram skin cells in vitro before using them for cloning.
This work is dependent on further funding and applications have/are being submitted to various funding agencies as well as venture capital groups.
Contributors
University of Adelaide
- Robinson Research Institute
- Adelaide Medical School
- School of Animal and Veterinary Sciences
References
- Nuclear Cloning, Stem Cells, and Genomic Reprogramming, Cloning and Stem Cells
- In vitro and in vivo characterisation of putative porcine embryonic stem cells, Cellular Reprogramming
- Isolation and in vitro characterisation of putative porcine embryonic stem cells from cloned embryos treated with Trichostatin A, Cellular Reprogramming
