Catalytic activity of metal clusters on surfaces
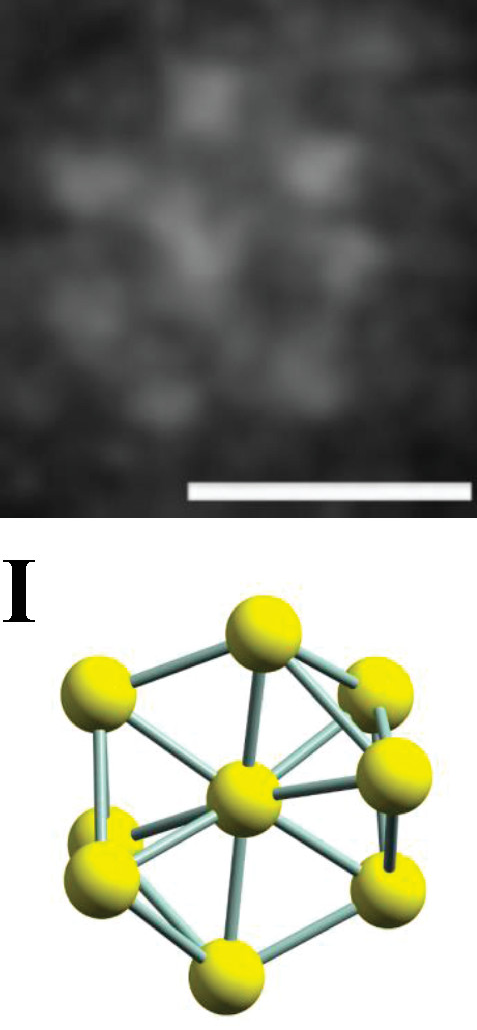
Figure shows the HR-TEM image of the Au9 cluster deposited on a titania nanosheet. Scale bar is 0.5 nm.
For any possible application of metal clusters as catalysts it is necessary to deposit them onto surfaces.
Therefore, it is the geometric and electronic (valence) structure of clusters at the surface that determines their interactions, and consequent reactivity, with molecules.
We are developing techniques to determine the geometric and electronic structure of size-specific clusters (e.g. Au9) deposited onto semiconductors surfaces (e.g. TiO2) and test their performance for photocatalytic water-spitting (H2 production) and CO2 reduction (hydrocarbon formation).
The characterisation studies involve the direct comparison between the physical and chemical properties displayed by isolated and deposited clusters to disentangle the surface effects.
Surface characterisation is undertaken via a combination of X-ray Photoelectron Spectroscopy (XPS), Scanning Tunnelling Microscopy (STM) and Metastable Induced Electron Spectroscopy (MIES). This also involves visits to the Australian Synchrotron to use Soft X-ray and Far-IR spectroscopies.
Combined with quantum chemical calculations, these techniques provide information about the interaction between each size-specific cluster and the titania surface. In addition, we use ultra-high resolution Transmission Electron Microscopy to directly observe, with atomic resolution, individual clusters on the surface.
We also experimentally measure the catalytic performance of these clusters-based systems using a specially-developed reactor systems.
Skills to be developed include operating simulated concentrated solar sources, high vacuum pumps and gas handling and detection systems, as well as learning to undertake advanced surface electron spectroscopy characterisation techniques and atomically-resolved, high resolution electron microscopy measurements. Quantum computational calculations can also be part of this project.
This work is done with collaborators at Flinders, Newcastle, Canterbury, (NZ), and Utah (USA) universities and could include visits to these labs.
Study in the Metal Cluster Laboratory
Professor Gregory Metha leads the Metal Cluster Laboratory which investigates how the properties of the metallic elements are affected by their size.
At the sub-nanometre size regime (< 1 nm), metal particles, called clusters, have chemical and physical properties that are markedly different from the bulk due to the dominance of quantum size effects.
Furthermore, each atom within the cluster is not 'locked' into place, allowing clusters to be able to move readily between various structural minima. Due to the size dependent variation of each cluster's electronic and geometric structure, the interaction of a specific sized metal cluster with a molecule (e.g. reactivity) is also unique.
Therefore, we consider cluster size to be the third dimension of the Periodic Table, which can be manipulated to produce new chemical species with novel chemical and physical properties that can explored and potentially exploited.
It has only been recently demonstrated that the size variable reactivity of metal clusters can be utilised to enhance catalytic activity. Metal clusters deposited onto surfaces, containing as few as several atoms, have been shown to induce catalysed activity at significantly lower temperatures compared with bulk metallic surfaces, and also demonstrate improved selectivity for particular reaction products.
This emerging field of research is known as nanocatalysis, where the properties depend on exactly how many atoms are in each cluster. The study and understanding of the underlying principles of these effects have the potential to provide a revolutionary methodology for developing next generation ultra-efficient catalysts.
Research within the Metal Cluster Laboratory involves the experimental and theoretical study of the chemical and physical properties of metal clusters, their deposition onto surfaces, and their chemical activity towards important molecules such as CO2, H2O and N2.
The research is broadly separated into 2 themes; (i) fundamental laser spectroscopic investigations of metal cluster systems isolated in the gas phase; and (ii) application of metal clusters as catalysts and photocatalysts.
Both themes are underpinned by computational studies to help explain the experimental data and provide insight into the strange nature of metal clusters.

