Complement receptor immunoglobulin - infection & immunity & inflammation
These immunopathology research projects examine the complement receptor immunoglobulin expression in human macrophages and its role in infection and immunity and inflammation.
Complement receptor immunoglobulin (CRIg) is expressed selectively in macrophages. Apart from its primary role in phagocytosis of complement opsonised bacteria, it has been recognised to mediate anti-inflammatory and immunosuppressive activities.
Our group has been conducting research to characterise the various isoforms of CRIg and the role which they play in infection and immunity and inflammation in an effort to provide new biomarkers and drug targets in these diseases.
To contribute to this goal we offer two projects suitable for honours research:
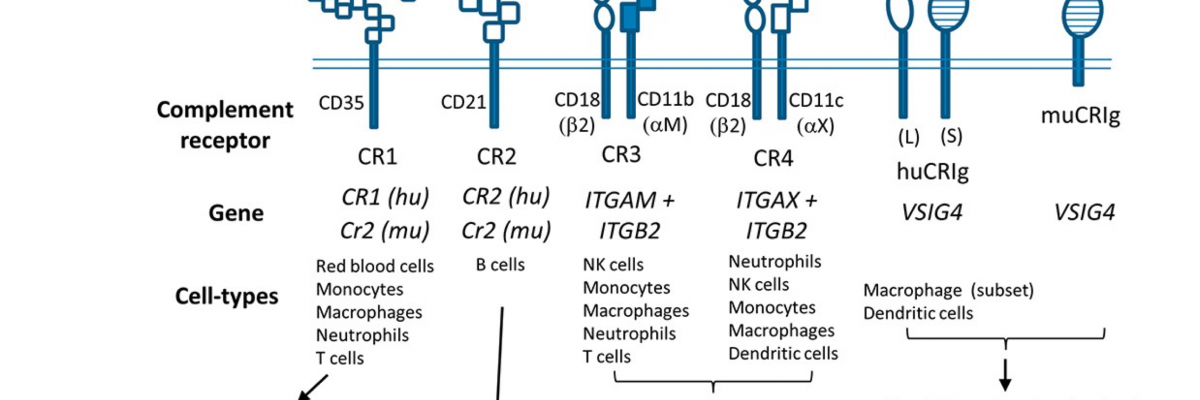
Figure 1. Characteristics, function and expression of complement receptors
Project 1
The aim of this research is to characterise the role of CRIg relative to the other complement receptors in bacterial phagocytosis and killing (Fig 1). To facilitate this work a human macrophage cell line (THP-1) model has been developed which can be differentiated into mature macrophages. Our Lab has shown that these macrophages express CRIg, CR3 and CR4 but not CR1 (Fig 1).
The initial results using our CD18 deficient THP-1 macrophages which do not express CR3 and CR4 has shown the retention of full phagocytosis and killing capacity of complement opsonised bacteria by the mutant THP-1 cells, suggesting that CRIg is the main phagocytosis receptor on these macrophages.
This unexpected finding will be explored further by knocking out VSIG4 in THP-1 cells to produce CRIg deficient macrophages, using the CRISPR CAS9 genome editing system. The cells will then be examined for phagocytosis and bacterial killing. The mechanisms involved in phagocytosis and microbial killing via CRIg will also be evaluated.
The structural domains of the five known types of complement receptors are depicted, together with their specificity of C3 fragments, the genes encoding them, their distribution amongst the different leucocyte types and their known functions. CR1, CR2 and CRIg are single transmembrane proteins with extracellular portions, transmembrane domains and cytoplasmic tails whereas CR3 and CR4 are transmembrane heterodimers of a common β2 integrin (CD18) chain and an α integrin chain, αM (CD11b) or αX (CD11c).
Murine CR1 and CR2 are derived from the same gene by alternative splicing whereas the human counterparts are encoded by 2 different genes. CR1 contains thirty short consensus repeats (SCR) and CR2 has fifteen SCR. CD18 contains four repeats and a Von Willebrand factor type A domain (lightly shaded oblong shape).
The α integrins contain, within their extracellular portions, seven FG-GAP repeats (rectangles) and a Von Willebrand factor type A domain. Two human CRIg isoforms, huCRIg(L) for the long form and huCRIg(S) for short, have been described.
Both isoforms contain an N-terminal ligand binding domain that belongs to the IgV-type of immunoglobulin domains (horizontal stripes). The long form of CRIg also contains a membrane proximal domain that is an IgC-type immunoglobulin domain. The function of this domain is unclear. The murine form, similar to CRIg(S), contains only the IgVtype of immunoglobulin domain but the cytoplasmic tail is shorter than that of huCRIg(S). The IgV domains are believed to be responsible for binding C3 fragments.
Project 2
Our group has just generated monoclonal antibodies to human and mouse CRIg. This project will endeavour to characterise these for potential use in western blots flow cytometry and ELISA assays.
Because it has been found that CRIg expression may serve as a useful biomarker in in cancer and inflammatory diseases, the characterisation of hybridomas producing appropriate mAb is desirable and will be defined as part of this project.
Since these antibodies have been directed to the region on CRIg which binds iC3b it is likely that they can be used to block the interaction between the ligand and the receptor.
This study will examine the ability of these mAb to block this interaction with the aim of developing these as tools to explore mechanisms but in addition for the development of therapeutics for use in cancer treatment.
The present project will explore the ability of these mAb to block the immunosuppressive properties of CRIg-expressing antigen presenting cells.
If you have an interest in a specific area of immunopathology, please contact Professor Tony Ferrante to discuss potential honours projects.

Supervisor
Research area: Development and genetic immunology, immunopathology
Recommended honours enrolment: Honours in Molecular and Biomedical Science
