Efficacy of a combination of TKI and a novel allosteric inhibitor asciminib in chronic myeloid leukaemia
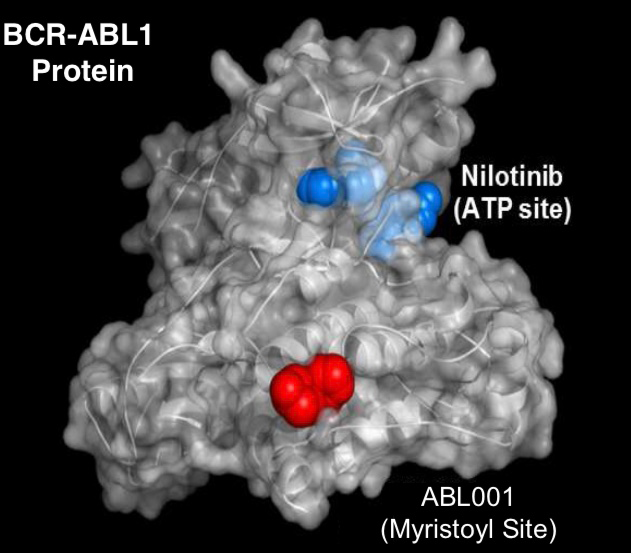
Clinical trials for the treatment of Chronic Myeloid Leukemia (CML) are currently underway using asciminib (ABL001).
The trials are using this allosteric inhibitor alone, and in combination with ATP-competitive tyrosine kinase inhibitors (TKIs: imatinib, nilotinib or dasatinib), to inhibit the constitutively active tyrosine kinase Bcr-Abl.
We will then examine mechanisms of treatment resistance in vitro, in an attempt to predict emergent disease resistance mechanisms that may arise.
The aims of this project are:
- Investigation of the synergistic effect of the combination of TKI and asciminib.
- Understand the signalling pathway changes in BCR-ABL+ cell lines and CML patient cells when combination therapy is given.
- Generation of resistant cell lines in the laboratory setting. This provides a useful tool for predicting and studying patient responses in vivo.
Understanding how these drugs work in synergy will enhance our ability to predict whether patients are likely to respond to combination therapy, and clarify ways to maximise synergism between these agents.
In this project, BCR-ABL1+ cell lines will be exposed long term to gradually increasing concentrations of asciminib in combination with TKI. Mechanisms of resistance will be interrogated during resistance development and once overt resistance is observed.
Join our cancer research program
The treatment of chronic myeloid leukaemia (CML) has been one of the most remarkable cancer success stories this century, heralding the widespread application of small molecules to target oncogenic kinases.
Insights from CML research in this era have provided guidance for the targeted therapy programs in many other cancers. The improvement in 10-year survival for CML patients from 20% in the 1990s to over 80% today has been achieved with the clinical application of tyrosine kinase inhibitor (TKI) therapy targeting Bcr–Abl.
Despite the improvements in outcomes achieved with TKI therapy, major challenges still confront the CML clinician. Transformation to blast crisis is still seen in -10%, similar numbers are resistant to all TKls and only -50% overall achieve deep molecular responses (DMR). Furthermore, most CML patients will remain dependent on TKI therapy for life with current approaches. As well as the massive cost burden, this long–term dependence on TKI therapy often leads to impaired quality of life and, in some cases, significant organ damage.
Pioneering work from the Bordeaux and Adelaide trial groups has shown that around half of the patients who achieve stable DMR can cease TKI therapy without evidence of molecular relapse, even with long-term follow-up ( defined as achieving treatment-free remission -TFR).
By contrast the other half have molecular evidence of recurrence, usually within 6 months of stopping, and have to restart TKI therapy. We have made excellent progress in understanding some of the key drivers of DMR and TFR and are already translating some of these findings into clinical trials to expand opportunity for these optimal outcomes.
This emerging knowledge will provide urgently needed criteria for safe TKI cessation and guide the design of future trials to maximise TFR with consequent major benefits for many CML patients. CML is projected to become the most prevalent leukaemia by 2040, so for the thousands of CML patients in Australia who are facing lifelong dependence on expensive and debilitating therapy, support for this work is critical.

Supervisors
Co-supervisors: Dr David Yeung | Dr Liu Lu
Research area: Cancer program - South Australian Health and Medical Research Institute
Recommended honours enrolment: Honours in Molecular and Biomedical Science
