Metabolic dependencies for improved therapeutic outcomes in chronic myeloid leukaemia
While the treatment of BCR-ABL1 driven chronic myeloid leukaemia (CML) with tyrosine kinase inhibitors (TKI) has been a success story of modern medicine, not all patients respond optimally, with a small but significant minority progressing to fatal blast crisis.
The inability of TKIs to eradicate leukaemic stem and progenitor cells (LSPC) provides a potential pool for subsequent relapse. This is exemplified by the dependence of most CML patients on lifelong TKI treatment, even in patients who achieve molecular responses.
Chronic Myeloid Leukemia smear
Paulo Henrique Orlandi Mourao (CC BY-SA 3.0)
Universally, the presence of an activated tyrosine kinase drives metabolic changes, and the perturbation of these metabolic pathways provides potential avenues for exploration of therapeutic interventions. Targeting metabolic pathways may potentiate the effect of TKI therapies particularly in the LSPC compartment.
The aim of the project will be to investigate the metabolic pathways that exists within normal haematopoietic cells (important knowledge to avoid toxicity), and in LSPC including cases sensitive and resistant to TKIs to enable a clear understanding of whether the metabolome of LSPC can be targeted to sensitise this pool to TKI therapy.
This novel and innovative approach will likely provide a combinational treatment strategy with greater efficacy than current approaches, specifically in CML blast crisis.
Join our cancer research program
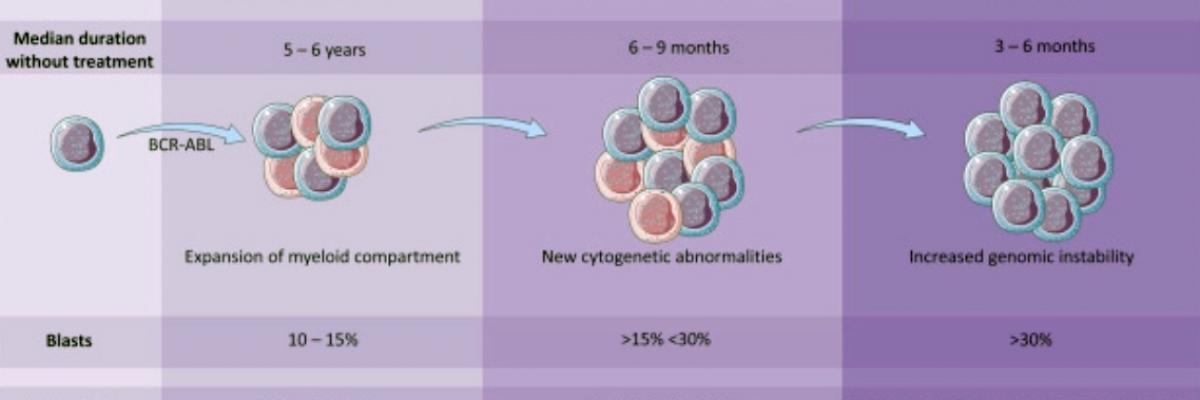
The treatment of chronic myeloid leukaemia (CML) has been one of the most remarkable cancer success stories this century, heralding the widespread application of small molecules to target oncogenic kinases.
Insights from CML research in this era have provided guidance for the targeted therapy programs in many other cancers. The improvement in 10-year survival for CML patients from 20% in the 1990s to over 80% today has been achieved with the clinical application of tyrosine kinase inhibitor (TKI) therapy targeting Bcr–Abl.
Despite the improvements in outcomes achieved with TKI therapy, major challenges still confront the CML clinician. Transformation to blast crisis is still seen in -10%, similar numbers are resistant to all TKls and only -50% overall achieve deep molecular responses (DMR). Furthermore, most CML patients will remain dependent on TKI therapy for life with current approaches. As well as the massive cost burden, this long–term dependence on TKI therapy often leads to impaired quality of life and, in some cases, significant organ damage.
Pioneering work from the Bordeaux and Adelaide trial groups has shown that around half of the patients who achieve stable DMR can cease TKI therapy without evidence of molecular relapse, even with long-term follow-up ( defined as achieving treatment-free remission -TFR).
By contrast the other half have molecular evidence of recurrence, usually within 6 months of stopping, and have to restart TKI therapy. We have made excellent progress in understanding some of the key drivers of DMR and TFR and are already translating some of these findings into clinical trials to expand opportunity for these optimal outcomes.
This emerging knowledge will provide urgently needed criteria for safe TKI cessation and guide the design of future trials to maximise TFR with consequent major benefits for many CML patients. CML is projected to become the most prevalent leukaemia by 2040, so for the thousands of CML patients in Australia who are facing lifelong dependence on expensive and debilitating therapy, support for this work is critical.

Supervisors
Co-supervisors: Dr Ilaria Pagani
Research area: Cancer program - South Australian Health and Medical Research Institute
Recommended honours enrolment: Honours in Molecular and Biomedical Science
