Synthesis using Nature’s catalysts; enzymes
Microorganisms such as bacteria represent the most diverse branch of life being found in every environment on earth capable of supporting life.
Bacteria that can grow in different environments have evolved to utilise and synthesise a broad range of chemicals. The utilisation of bacterial enzymes for synthesis has many advantages over chemical systems (mild conditions, improved selectivity, less waste).
Honours projects in this area involve the isolation and study new enzymes from bacteria of interest or taking existing enzymes and adapting them for chemical synthesis. This includes utilising rational and directed evolution protein engineering techniques (see Nobel Prize in Chemistry 2018 to Prof. Frances Arnold).
The crystal structure of the enzymes are also determined which allows the investigation of their properties in more detail and their rational modification.
Project aims
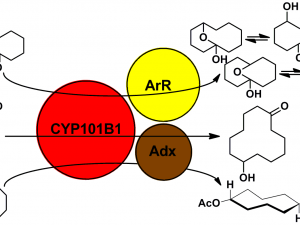
The aim of these projects is to delineate the function of enzymes from bacteria which have related but different amino acid sequences. The goal is to find molecules which bind with a preference for selective activity, or to adapt the enzyme to achieve this, and to determine the products.
A number of different substrate classes will be targeted including terpenoid compounds to generate flavour and fragrance compounds, alkanes and halogenated aromatics for bioremediation.
Alternatively we can also alter the product selectivity by mutating the enzyme or by chemically adding protecting groups to modify the substrate binding orientation.
Techniques used
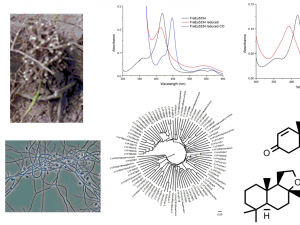
In the laboratory, honours students employ a number of chemical and/or biochemical techniques. Organic and analytical chemistry techniques are used for the production, isolation and identification of hydroxylated organics from enzymatic turnovers and whole-cell reactions via HPLC, GC, GC-MS and NMR.
Genome analysis and molecular biology (e.g. gene cloning, rational mutagenesis and directed evolution), protein production using Escherichia coli and protein purification are performed.
The inorganic metal centres of the enzymes and the electron transfer proteins are analysed using UV/Vis and other spectroscopies. Structural studies (X-ray crystallography) of the proteins are undertaken. The structures provide important information on how the enzymes function and the protein-protein interactions which control electron transfer. Projects can therefore be designed to be compatible for those with an interest in chemical synthesis/analysis or in the biochemical aspects of the work.
Study honours in medicinal chemistry
The Bell Group investigates the enzyme complement of metabolically diverse microorganisms (microbiology) to discover functional systems with applications in biocatalysis (chemistry) and to understand their physiological function e.g. electron transfer proteins and enzymes in involved in secondary metabolism (biochemistry).
This research is critical to understand the role of enzyme across a broad range of disciplines including medicine (drug metabolism and interactions), fine chemical synthesis (flavour and fragrance and drug metabolite generation) and bacterial secondary metabolism (synthesis of complex bioactive natural products).
Supervisor
- Associate Professor Stephen Bell
- Research area: Biocatalysis, organic chemistry, microbiology, molecular biology, synthetic biology
- Recommended honours enrolment: Honours in Chemistry