Understanding the mechanism of enzyme catalysed reactions of importance in drug metabolism
The goal of this project is to understand the mechanism by which the major family of enzymes involved in drug metabolism catalyse oxidative transformations.
Such reactions are critical in both drug metabolic and biosynthetic pathways but are often poorly understood because of the difficulty of working with the enzymes and substrates involved. A better understanding their mechanism will allow one to predict and utilise their occurrence in drug metabolism, exploit such oxidations in biotechnological-based fine chemical synthesis and inhibit them in key biosynthetic pathways to develop novel chemotherapeutics.
Project aims
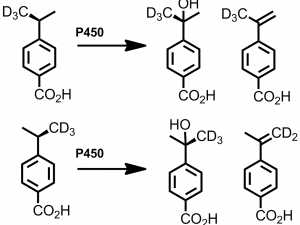
Understand the mechanism of the reactions catalysed by these enzymes. Establish the generality of the reactions and determine the structural requirements of the substrate. Work out the presence/absence and nature of intermediates via mechanistic probes.
Understand the role that the protein residues and the enzyme-substrate interactions play by: determining the substrate-bound X-ray crystal structures with compounds that undergo these reactions.
This information is combined and extended to human drug metabolising enzymes to inform the design of better drug molecules.
Techniques used
In the laboratory, honours students employ a number of chemical and/or biochemical techniques. Organic and analytical chemistry techniques are used for the production, isolation and identification of hydroxylated organics from enzymatic turnovers and whole-cell reactions via HPLC, GC, GC-MS and NMR.
Genome analysis and molecular biology (e.g. gene cloning, rational mutagenesis and directed evolution), protein production using Escherichia coli and protein purification are performed.
The inorganic metal centres of the enzymes and the electron transfer proteins are analysed using UV/Vis and other spectroscopies. Structural studies (X-ray crystallography) of the proteins are undertaken. The structures provide important information on how the enzymes function and the protein-protein interactions which control electron transfer. Projects can therefore be designed to be compatible for those with an interest in chemical synthesis/analysis or in the biochemical aspects of the work.
Study honours in medicinal chemistry
The Bell Group investigates the enzyme complement of metabolically diverse microorganisms (microbiology) to discover functional systems with applications in biocatalysis (chemistry) and to understand their physiological function e.g. electron transfer proteins and enzymes in involved in secondary metabolism (biochemistry).
This research is critical to understand the role of enzyme across a broad range of disciplines including medicine (drug metabolism and interactions), fine chemical synthesis (flavour and fragrance and drug metabolite generation) and bacterial secondary metabolism (synthesis of complex bioactive natural products).
Supervisor
- Associate Professor Stephen Bell
- Research area: Medicinal chemistry, structural biology, enzyme mechanisms
- Recommended honours enrolment: Honours in Chemistry