Metal-organic Frameworks as Chemical Reaction Flasks
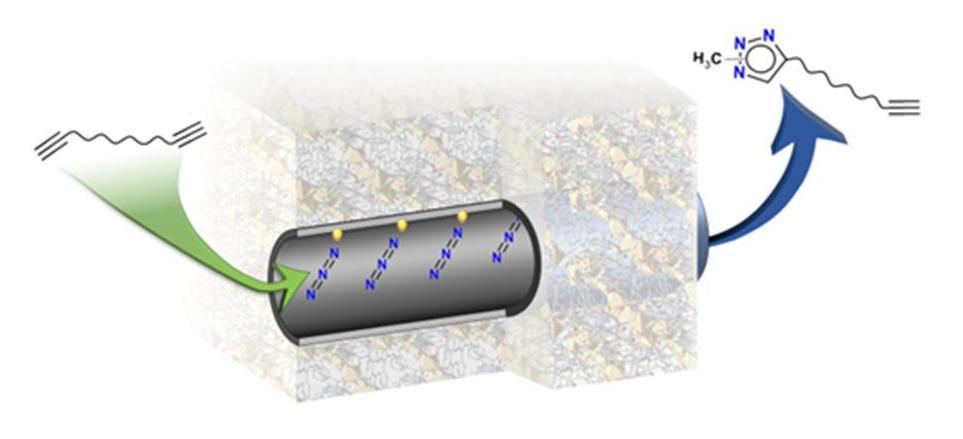
We have recently clearly articulated the mechanism by which a chemoselective click chemistry can be carried out on dialkyenes.
Chemoselective organic transformations are essential for atom efficient synthesis of complex molecules.
Equally, synthetic chemists are often required to utilise toxic reagents in synthesis. Metal-organic Frameworks (MOFs) can be designed to promote chemoselective reactions or to operate as mechanical protecting groups.
We have recently clearly articulated the mechanism by which a chemoselective click chemistry can be carried out on dialkyenes (see Figure).[1] Is this a broader concept that can be extended to other reactions or more complex substrates? Can MOFs also be used to provide toxic reagents in a controlled manner?
This project will explore the use of MOFs to deliver toxic or difficult to handle reagents for synthesis. Design of bespoke MOFs capable of being loaded with reagents will be undertaken and the application of these materials for use in synthesis will investigated.
- M. T. Huxley et al. J. Am. Chem. Soc., 2018, 140, 6416.
Study honours in chemistry
The Sumby Doonan Group are a chemistry research group based at the University of Adelaide, investigating the design, synthesis and applications of porous materials.

Supervisors
Co-supervisors: Professor Christian Doonan
Research area: Macromolecular chemistry, metal-organic frameworks, porous materials, catalysis, organometallic chemistry - Centre for Advanced Nanomaterials
Recommended honours enrolment: Honours in Chemistry
