Exploring the fundamentals of photo-induced chemical reaction mechanisms
Use high-speed lasers to initiate a water-splitting reaction to probe products for analysis.
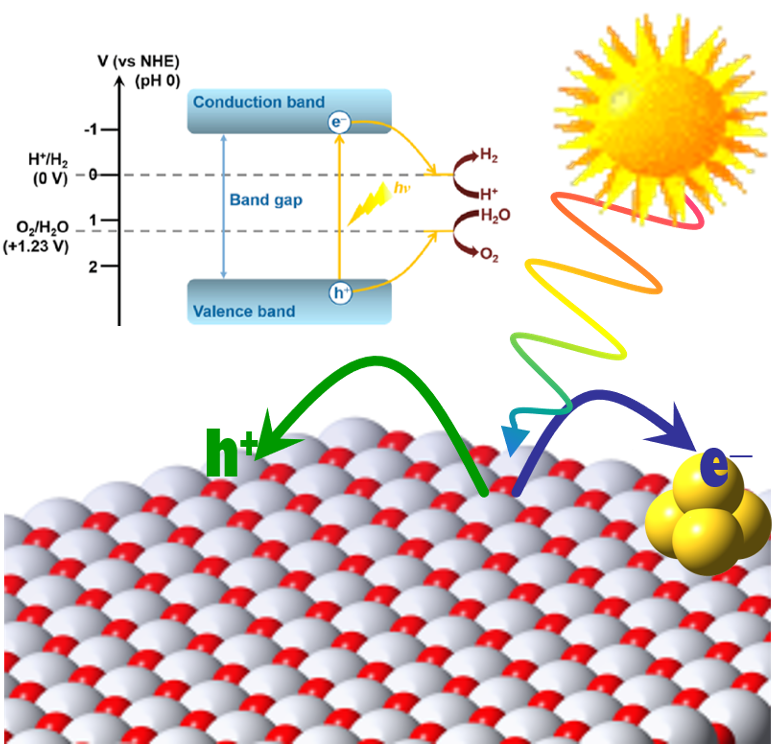
Photocatalysis involves both electron and hole transfer, as well as molecular re-arrangement on a surface.
Photo-induced chemical reactions convert solar energy into chemical energy that can drive chemical reactions. This makes the photo-induced reactions an essential element for a ‘green’ future.
These chemical reactions require a catalyst, termed a photocatalyst, that reduces the activation energy of the reactions and enable the photons from the sunlight to be able to drive the reaction.
However, the current state of the photo-induced reactions requires significant amount of improvement before they can be put to daily use.
Photo-induced chemical reactions are used to accomplish various objectives such as:
- water and air purification;
- self-cleaning and self-sterilising surfaces;
- degradation of volatile compounds;
- eco-friendly surface treatments; and
- water-splitting.
Our current focus is on the water-splitting reaction. Hydrogen is considered to be the fuel of our ‘green’ future. However, green hydrogen needs to be cheaper before it can be adopted commercially to displace fossil fuels.
Understanding the individual steps that make up the photocatalytic reaction mechanism can reveal the factors that can make the process more efficient for hydrogen production.
We have built a novel experiment consisting of a modified TOF-mass spectrometer which is being utilised to study the molecular re-arrangements involved in the later parts of the reaction. High-speed lasers are used to initiate the water-splitting reaction and to probe the products for analysis.
Skills in operating high energy lasers, high vacuum pumps and HV power supplies will be developed as well as learning to undertake advanced quantum computational calculations and simulations.
Study in the Metal Cluster Laboratory
Professor Gregory Metha leads the Metal Cluster Laboratory which investigates how the properties of the metallic elements are affected by their size.
At the sub-nanometre size regime (< 1 nm), metal particles, called clusters, have chemical and physical properties that are markedly different from the bulk due to the dominance of quantum size effects.
Furthermore, each atom within the cluster is not 'locked' into place, allowing clusters to be able to move readily between various structural minima. Due to the size dependent variation of each cluster's electronic and geometric structure, the interaction of a specific sized metal cluster with a molecule (e.g. reactivity) is also unique.
Therefore, we consider cluster size to be the third dimension of the Periodic Table, which can be manipulated to produce new chemical species with novel chemical and physical properties that can explored and potentially exploited.
It has only been recently demonstrated that the size variable reactivity of metal clusters can be utilised to enhance catalytic activity. Metal clusters deposited onto surfaces, containing as few as several atoms, have been shown to induce catalysed activity at significantly lower temperatures compared with bulk metallic surfaces, and also demonstrate improved selectivity for particular reaction products.
This emerging field of research is known as nanocatalysis, where the properties depend on exactly how many atoms are in each cluster. The study and understanding of the underlying principles of these effects have the potential to provide a revolutionary methodology for developing next generation ultra-efficient catalysts.
Research within the Metal Cluster Laboratory involves the experimental and theoretical study of the chemical and physical properties of metal clusters, their deposition onto surfaces, and their chemical activity towards important molecules such as CO2, H2O and N2.
The research is broadly separated into 2 themes; (i) fundamental laser spectroscopic investigations of metal cluster systems isolated in the gas phase; and (ii) application of metal clusters as catalysts and photocatalysts.
Both themes are underpinned by computational studies to help explain the experimental data and provide insight into the strange nature of metal clusters.

